Oral Biofilm: What, Where, How & Why
- Feb 12, 2025
- 9 min read
Updated: Sep 15, 2025
Even in a healthy oral environment, there is a diverse population of microorganisms such as viruses, fungi, and bacteria [5]. This grouping of microorganisms is collectively known as the oral microbiome, which is discussed in detail in another blog post – find it here.
Today, we will look closer at a specific aspect of the oral microbiome that contributes significantly to periodontal diseases – oral biofilm.
The bacteria that live in the oral microbiome exist mostly in layers of biofilm, which are sticky, dense communities that attach to various surfaces within the oral cavity, such as teeth, gingival tissue, mucosal surfaces, and dental implant materials [2].
But how does it get there, and why should you care?
This discussion gets nerdy pretty quickly (which I love), but let’s start from the beginning.
What is biofilm?
Oral biofilm is a highly complex and specialized community of bacteria that organizes itself into protective structures on various surfaces in the mouth [12]. In health, oral biofilm is composed of commensal (i.e., beneficial) bacterial species that are harmless to the host [12].
However, biofilm’s innate protective structure makes the bacterial communities more resistant to external physical and chemical agents [12]. If left undisturbed, the growth and maturation of biofilm causes a shift towards the proliferation of pathogenic bacterial species. The resulting imbalance of commensal and pathogenic microbial communities is known as dysbiosis.
[15]

Overall, biofilm is a major initiating factor of disease in the oral cavity due to the harmful pathogens within biofilm rapidly increasing in a short amount of time.
It only takes about 24 hours for oral biofilm to form after mechanical removal, such as brushing and flossing. Undisturbed biofilm then mineralizes into calculus if left undisturbed for 10-20 days [3].
With a continued shift towards a dysbiotic environment, the risk for the development and progression of periodontal diseases increases significantly.
Where is biofilm found?
Biofilm is a sticky layer of bacteria with a self-produced extracellular matrix designed to protect the biofilm from external disruption. Because of this, biofilm is hardest to remove — and therefore the most protected — in areas where access is limited. However, understanding where biofilm is most likely to be present is essential for effective removal.
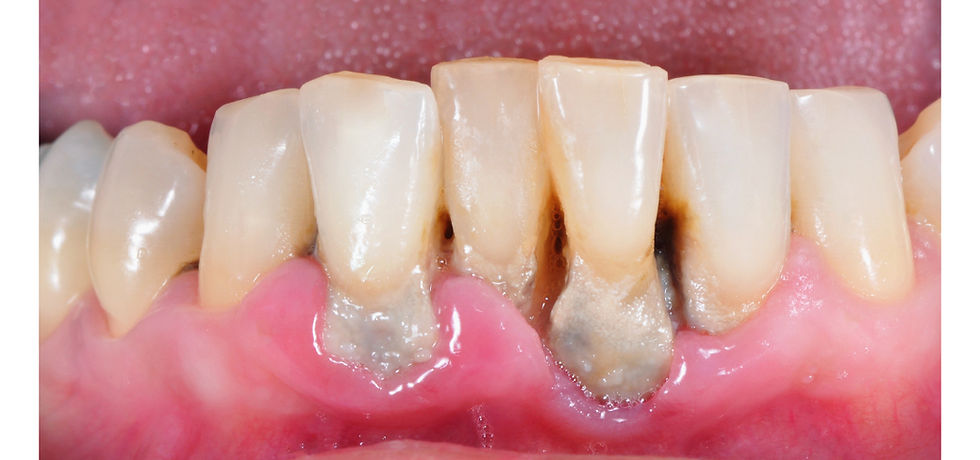
Dentally speaking, interproximal spaces, around dental restorations without smooth margins, near the gingival margin (i.e., the gum line), and the tongue typically have the most biofilm accumulation.

In the early stages, biofilm on teeth is invisible. When left undisturbed, it begins to appear as a white to yellow film as depicted in the image below. Once oral biofilm has matured into thicker layers and is visible to the naked eye, it becomes known as dental plaque.
[14]

Dental healthcare providers may utilize disclosing solutions to help put color to oral biofilms, which allows not only the provider but also the patient to see areas frequently missed during home care routines.
Understanding disclosing solution
Light pink = biofilm present for 24 hours or less
Dark blue/purple = biofilm present for greater than 48 hours
Light blue = areas of acidic biofilm, which contribute to dental decay

For optimal oral and systemic health, oral biofilms should be removed routinely with habits such as flossing, brushing, water flossing, tongue scraping, etc.
Use of disclosing solutions at home is a great adjunct to improving oral care routines for both children and adults.
Check out my recommended at-home disclosing solution products here
*Amazon Associate links
How does biofilm form, grow & communicate?
--- This is where we get nerdy! ---
The formation of biofilm is a complex process that begins via the formation of a thin layer known as a dental pellicle that forms immediately on tooth surfaces after cleaning, acting as the initial interface for bacteria to adhere and form biofilm. The dental pellicle layer is composed of salivary proteins, glycoproteins, and other macromolecules [13]. From this, the formation of more mature biofilm begins when adequate biofilm disruption does not occur.
The primary cause for the accumulation of dysbiotic biofilm on oral surfaces is poor oral hygiene [12].
On a simple level, oral biofilm development consists of phases with different bacterial species as protagonists [12]. As biofilm matures through specific stages of development, pathogenicity and virulence increase, leading to an increase in an inflammatory response both locally and systemically.
Biofilm Formation in Phases [12]
In the initial phase, pioneer bacterial species act as receptors to other bacterial species known as early colonizers, which take over in the second phase. With these groups of pioneer bacteria and early colonizers present, biofilm begins to form supragingivally through aggregation and co-aggregation (i.e., binding) mechanisms.
As biofilm matures and continues to grow, late colonizing bacteria build a more dense network of links to those created in previous phases. These late colonizing biofilm pathogens typically form subgingivally and are composed of gram-negative anaerobic bacteria, which play a significant role in the pathogenesis of periodontitis.
[12]

As noted above, bacterial species within oral biofilm produce extracellular polysaccharides (EPS), which act as the structural backbone of the biofilm. The biofilm matures as more layers of EPS are produced, which creates a three-dimensional mushroom-like structure [1].
The protective EPS layers aid in water retention, prevent desiccation and attack by harmful agents, provide nutrient storage, and retain necessary extracellular enzymes [1]
EPS are known for their ability to tolerate antibiotics, host defense systems, and other environmental stresses, which contribute to the chronic infectious nature of biofilm and therefore pose a serious global health concern [4]
Summary of phases in biofilm formation
First phase: Pioneers are responsible for initial attachment to the surfaces of the tooth
Second phase: Early colonizers initiate the maturation of the supragingival biofilm
Third phase: Later colonizers cause the formation of subgingival biofilm
As more pathogenic bacteria overtake the biofilm matrix, there is a continuous decrease in commensal bacterial growth, and the shift toward a dysbiotic oral environment continues
Beyond what pathogens are present at which phase, it’s also important to understand the mechanisms of action for how biofilm forms through various stages of maturity.
Biofilm Formation in Stages [5]
Acquired pellicle formation: Formation of a glycoprotein film that forms on the tooth surface in response to contact with saliva
Initial adhesion: Bacterial pathogens make an initial attachment to the surface of teeth via the acquired pellicle salivary film of glycoproteins, which serves as a substrate for attachment of microorganisms
Colonization (coaggregation): Organization of microorganisms in colonies according to species
Growth (maturation): Increase in the number and species of microorganisms in biofilm
Dispersion (diffusion): After the biofilm is organized and well established, particles of the biofilm detach, migrate, and adhere to other surfaces within the oral cavity
[6]

Summary of phases & stages in biofilm formation [6]
Salivary glycoprotein films form on the tooth surface and act as the underlying substrate of the oral biofilm matrix
Adhesion of pioneer, initial colonizers atop the acquired pellicle protein layer begins the biofilm formation process
As biofilm goes undisturbed, growth and maturation of the biofilm occur, and late colonizers continue to increase in prevalence and virulence within the biofilm matrix
Pathogens within the biofilm are protected by an extracellular polysaccharide (EPS) coating, which aids in the maintenance of ideal growth parameters for the biofilm
Particles of the mature biofilm break free, allowing for the initiation of a new cycle of biofilm formation to begin on a new surface within the oral cavity
Taking it to a deeper level, let’s discuss how bacterial pathogens organize and communicate to ensure their continued growth.
Communication via Quorum Sensing
Quorum sensing (QS) is a form of cell-to-cell communication between bacterial species that allows bacteria to respond with gene expression changes to adapt to the changing environmental conditions that arise as a consequence of the increased number of bacteria and decreased availability of nutrients [11].
Once a biofilm has grown to a particular cell density, the bacteria within the matrix communicate with each other via signaling molecules called autoinducers (AIs) [11].
[12]

Bacterial populations within a biofilm are able to change their behavioral patterns based on variations in the physiological and biochemical parameters of nearby bacterial populations [1].
Overall, QS is a form of communication within and among biofilm formations that allows for coordinated gene expressions to enhance biofilm stability, virulence factors, pathogenesis, antibiotic resistance, and more [1].
QS Induction for Gram-Positive and Gram-Negative Bacteria
Although the outcome is similar, there are key differences between QS for gram-positive and gram-negative bacterial species.
—Gram-positive bacteria communication
Follows a two-component system in which membrane-bound sensor receptors and cytoplasmic transcription factors direct alterations in gene expression.
Autoinducer signaling molecule: oligopeptides — autoinducer peptides (AIP)
Due to gram-positive bacteria having a thick peptidoglycan layer in their cell wall, the autoinducer signaling molecules for gram-positive bacteria, AIPs, require specific receptors on the target cell surface in order to be detected [8]
Mechanism of communication
As bacterial populations grow, more AIPs are produced and released into the extracellular environment [9]
When a critical concentration of AIPs is reached, they bind to specific receptor proteins on the target bacterial cell membrane [9]
The binding of AIPs triggers a phosphorylation cascade within the cell and activates a response regulator protein [11]
This activated response regulator binds to DNA, leading to the transcription of specific genes responsible for desired behaviors [11]
[7]

— Gram-negative bacteria communication
Follows a complex system that allows bacteria to synchronize their biofilm-forming activities based on the local population density.
Autoinducer signaling molecule: acyl-homoserine lactone (AHL)
Although gram-negative bacteria have an additional outer membrane, AHLs can easily diffuse across the outer membrane due to their small size [8]
Key communicators in AHL-based QS systems
LuxI-type protein functions as a cytoplasmic AHL synthase, responsible for synthesizing AHL molecules
LuxR-type protein acts as an AHL-responsive DNA-binding transcriptional regulator
Mechanism of communication
As bacterial population density within biofilm increases, the concentration of AHL signals also increases
Once a threshold concentration of AHL is reached, LuxR-type transcription proteins bind to AHL signals, forming a LuxR/AHL complex
This LuxR/AHL complex then binds to specific DNA sequences known as lux boxes
The binding of a LuxR/AHL to a lux box alters gene expression and leads to coordinated regulation of various bacterial functions
[7]

Summary of QS mechanisms in biofilm formation & maturation
The quorum-sensing signaling mechanism in bacteria is one in which bacteria sense the density of the population and adjust gene expression accordingly [10]. This allows bacteria to control the metabolic and physiologic properties involved in bacterial existence, pathogenesis, and virulence [1].
Overall, the biological purpose of QS is believed to be related to the inflammatory response that occurs with bacterial infections. Essentially, as a bacterial infection continues to mature and spread, effective communication in and between the bacterial cells becomes increasingly necessary for bacterial survival [11].
In terms of oral health, QS is a regulating mechanism for gram-positive and gram-negative bacterial species present in oral biofilms, and it renders oral biofilms and pathogens resistant to various drugs and treatment regimes [1], especially as they mature in their bacterial strains.
Why should you care about biofilm?
In recent years, dentistry has developed a better understanding of the role oral biofilms play in the initiation and progression of oral diseases. Because of this, the removal and management of oral biofilm has become of greater focus for dental healthcare providers as a way to help prevent and control disease.
Oral biofilm formation is a rapid process that, if not interrupted at an early stage with effective daily oral hygiene routines, results in an increase in the proliferation of more pathogenic, late-stage bacterial species.
The prolonged exposure to late-stage bacterial species results in the initiation and progression of an inflammatory response, which leads to the development of oral diseases such as gingivitis and periodontitis. For many, undisturbed, long-term exposure to pathogenic oral biofilms also leads to dental decay.
Additionally, with an increase in the proliferation of more pathogenic bacterial species, both local and systemic inflammatory responses are initiated.
Historically, years of research support the idea that poor oral hygiene leads to a systemic inflammatory state and contributes to an increased risk of developing various diseases such as cardiovascular diseases, metabolic diseases, obesity, autoimmune disorders, some types of cancer, and more [12]
We’ll discuss the impacts poor oral health has on each body system in later posts, but for now, know that oral health and systemic health are extremely interconnected. You cannot be healthy systemically if pathological processes are happening within the mouth.
Talk soon,
Liz Laney, BSDH, RDH
Oral-Systemic RDH & Educator

Sources
Arya S, Usha R. Bioprospecting and exploration of phytochemicals as quorum sensing inhibitors against Cariogenic Dental Biofilm. Journal of Pure and Applied Microbiology. 2024;18(1):100-117. doi:10.22207/jpam.18.1.10
Bertolini M, Costa RC, Barão VAR, Villar CC, Retamal-Valdes B, Feres M, Silva Souza JG. Oral Microorganisms and Biofilms: New Insights to Defeat the Main Etiologic Factor of Oral Diseases. Microorganisms. 2022; 10(12):2413. https://doi.org/10.3390/microorganisms10122413
Chamberlain NR, Phillips PL. Dental plaque be bad! A. T. Still University of Health Sciences. October 2016. https://www.atsu.edu/faculty/chamberlain/mosdoh/dentalbiofilmplaguegonebad.htm#:~:text=A%20diverse%20collection%20of%20oral,dental%20biofilm%20to%20form%20again.
Das A, Patro S, Simnani FZ, et al. Biofilm modifiers: The disparity in paradigm of oral biofilm ecosystem. Biomedicine & Pharmacotherapy. 2023;164:114966. doi:10.1016/j.biopha.2023.114966
Dental Biofilm. Laboratorios KIN. https://www.kin.es/en/patologias/biofilm-dental/#:~:text=All%20foods%20rich%20in%20sugar,accumulation%20on%20the%20tooth%20enamel.
Hao Y, Huang X, Zhou X, et al. Influence of Dental Prosthesis and Restorative Materials Interface on Oral Biofilms. Int J Mol Sci. 2018;19(10):3157. Published 2018 Oct 14. doi:10.3390/ijms19103157
Johnson C. Quorum Sensing and Biofilm Formation: The Impact on Food Safety and Quality. QualiTru Sampling Systems. https://qualitru.com/quorum-sensing-and-biofilm/.
Miller MB, Bassler BL. Quorum sensing in bacteria. Annual Review of Microbiology. 2001;55:165-199. doi:10.1146/annurev.micro.55.1.165
Monnet V, Gardan R. Quorum‐sensing regulators in gram‐positive bacteria: ‘cherchez le peptide .’ Molecular Microbiology. 2015;97(2):181-184. doi:10.1111/mmi.13060
Otto M, Dickey SW, Wolz C. Editorial: Quorum-sensing in gram-positive pathogens – mechanisms, role in infection, and potential as a therapeutic target. Frontiers in Cellular and Infection Microbiology. 2023;13. doi:10.3389/fcimb.2023.1236705
Piewngam P, Chiou J, Chatterjee P, Otto M. Alternative approaches to treat bacterial infections: targeting quorum-sensing. Expert Rev Anti Infect Ther. 2020;18(6):499-510. doi:10.1080/14787210.2020.1750951
Polizzi A, Donzella M, Nicolosi G, Santonocito S, Pesce P, Isola G. Drugs for the Quorum Sensing Inhibition of Oral Biofilm: New Frontiers and Insights in the Treatment of Periodontitis. Pharmaceutics. 2022; 14(12):2740. https://doi.org/10.3390/pharmaceutics14122740
Schulz, A., Lang, R., Behr, J. et al. Targeted metabolomics of pellicle and saliva in children with different caries activity. Sci Rep 10, 697 (2020). https://doi.org/10.1038/s41598-020-57531-8
Sinclair L. The importance of controlling bacterial biofilm re-growth in patients with gingivitis. Colgate Professional. December 2021. https://www.colgateprofessional.com/dentist-resources/periodontics/importance-of-controlling-bacterial-biofilm-regrowth.
Yuan S, Fang C, Leng WD, et al. Oral microbiota in the oral-genitourinary axis: identifying periodontitis as a potential risk of genitourinary cancers. Mil Med Res. 2021;8(1):54. Published 2021 Sep 29. doi:10.1186/s40779-021-00344-1
%20-%20White_edited.png)


Comments